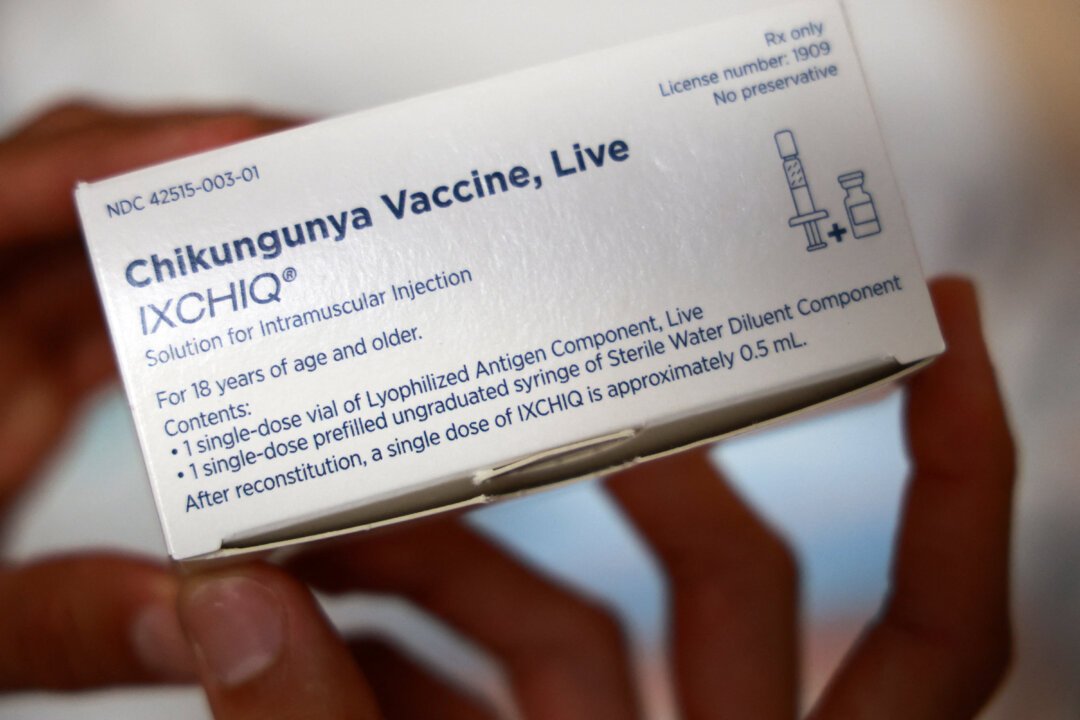
The Food and Drug Administration (FDA) has temporarily suspended the administration of a chikungunya virus vaccine for a segment of the adult population. This decision, announced on June 5th, follows reports of serious adverse events linked to the vaccination.
By early May, seventeen serious adverse events, including two fatalities, had been reported among recipients of the chikungunya vaccine. In a Journal of the American Medical Association article, Dr. Vinay Prasad, the FDA’s leading vaccine official, along with two colleagues, explained the agency’s action.
The FDA’s pause, they stated, is a crucial step to thoroughly investigate these reported events and uncover any potentially unreported adverse reactions. A key element of this investigation will involve determining a causal relationship between the reported events and the vaccine itself.
This requires a comprehensive analysis of various factors, including whether individuals receiving the vaccine also had a concurrent chikungunya infection. The FDA’s thorough investigation aims to ensure the safety and efficacy of the vaccine before resuming its administration.